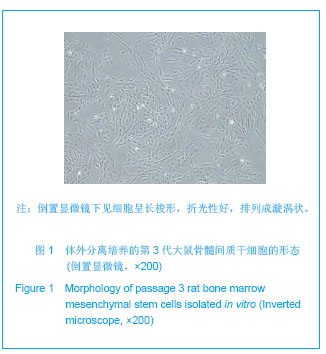
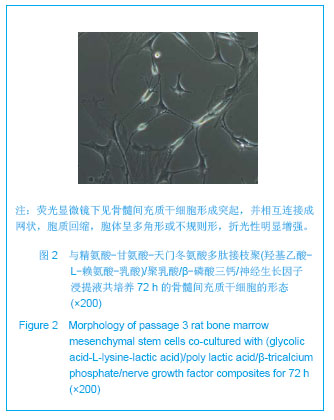
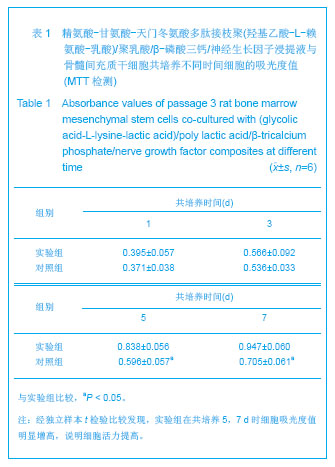
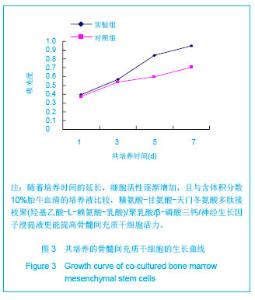
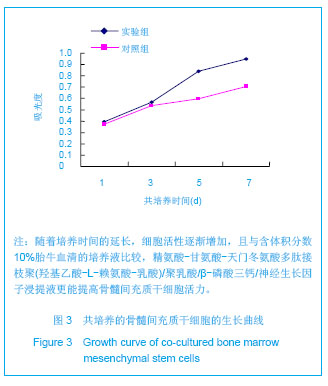
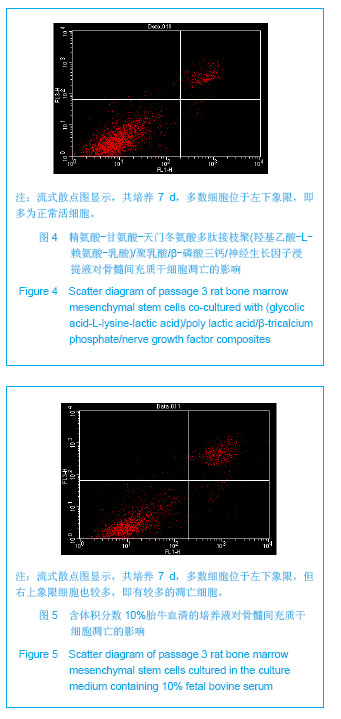
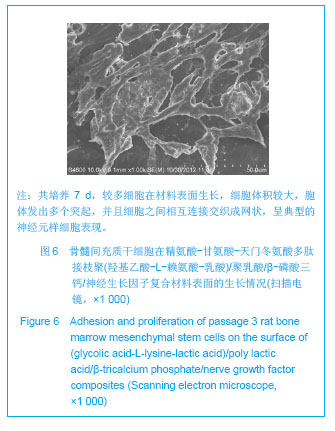
| [1]Tan A, Rajadas J, Seifalian AM. Biochemical engineering nerve conduits using peptide amphiphiles. J Control Release. 2012;163(3):342-352.[2]Nectow AR, Marra KG, Kaplan DL. Biomaterials for the development of peripheral nerve guidance conduits. Tissue Eng Part B Rev. 2012;18(1):40-50.[3]Pabari A, Yang SY, Mosahebi A, et al. Recent advances in artificial nerve conduit design: strategies for the delivery of luminal fillers. J Control Release. 2011;156(1):2-10.[4]Wang Y, Zhao Z, Ren Z, et al. Recellularized nerve allografts with differentiated mesenchymal stem cells promote peripheral nerve regeneration. Neurosci Lett. 2012;514(1): 96-101.[5]Yam QJ, Li SP, Yin YX, et al. Zhongnan Daxue Xuebao: Ziran Kexue Ban. 2008;39(6):1190-1195.严琼娇,李世普,殷义霞,等.RGD多肽接枝聚(乳酸-羟基乙酸-L-赖氨酸)的制备与表征[J].中南大学学报:自然科学版,2008,39(6): 1190-1195.[6]The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.中华人民共和国科学技术部.关于善待实验动物的指导意见. 2006-09-30.[7]Hu H, Huang JF, Zhang WC, et al. Huanan Guofang Yixue Zazhi. 2012;26(6):532-535.胡辉,黄继锋,张伟才,等.神经营养因子体外诱导鼠骨髓间充质干细胞分化为神经样细胞[J].华南国防医学杂志,2012,26(6): 532-535.[8]Yu C, Qiu T, Yan QJ, et al. Wuhan Ligong Daxue Xuebao. 2011;33(5):59-62.余畅,邱彤,严琼娇,等.PNGF复合材料对PC12细胞GAP43和β-TubulinII基因的调控[J].武汉理工大学学报,2011,33(5):59-62.[9]Zhang W, Yang ZX, Yi HL, et al. Zhongguo Jiaoxing Waike Zazhi. 2011;19(11):939-942.张伟,杨宗德,易红蕾,等.聚乳酸-聚羟基乙酸神经导管和骨髓间充质干细胞构建组织工程神经[J].中国矫形外科杂志, 2011, 19(11):939-942.[10]Cheng F, Hao HY, Tian HP, et al. Nanjing Yike Daxue Xuebao: Ziran Kexue Ban. 2010;30(1):54-58.程峰,郝怀勇,田和平,等.体外诱导骨髓间充质干细胞向神经细胞分化及凋亡的研究[J].南京医科大学学报:自然科学版,2010, 30(1): 54-58.[11]Gu X, Ding F, Yang Y, et al. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog Neurobiol. 2011;93(2):204-230.[12]Ichihara S, Inada Y, Nakamura T. Artificial nerve tubes and their application for repair of peripheral nerve injury: an update of current concepts. Injury. 2008;39 Suppl 4: 29-39.[13]Takagi T, Kimura Y, Shibata S, et al. Sustained bFGF-release tubes for peripheral nerve regeneration: comparison with autograft. Plast Reconstr Surg. 2012;130 (4):866-876.[14]Xiang J, Huang JF, Li DZ, et al. Zhongguo Zuzhi Gongcheng Yanjiu. 2012;16(21):3801-3805.向杰,黄继锋,李德忠,等.新型仿生人工神经导管诱导犬周围神经损伤再生过程的组织学观察[J].中国组织工程研究, 2012, 16(21): 3801-3805.[15]Zhang YQ, He LM, Xing B, et al. Neurotrophin-3 gene-modified Schwann cells promote TrkC gene-modified mesenchymal stem cells to differentiate into neuron-like cells in poly(lactic-acid-co-glycolic acid) multiple-channel conduit. Cells Tissues Organs. 2012;195(4):313-322.[16]Cuevas P, Carceller F, Garcia-Gómez I, et al. Bone marrow stromal cell implantation for peripheral nerve repair. Neurol Res. 2004;26(2):230-232.[17]Zhang PX, Jiang BG, He XJ, et al. Zhonghua Shouwaike Zazhi. 2004;8(20):200-202.张培训,姜保国,何湘君,等.骨髓间充质干细胞向雪旺细胞分化的体内外实验研究[J].中华手外科杂志,2004,8(20):200-202.[18]Xu LQ, Sui J, Ding Y, et al. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2007;11(7):1243-1246.徐龙强,隋静,丁钰,等.大鼠骨髓间充质干细胞体外培养转化为神经细胞的实验[J].中国组织工程研究与临床康复,2007,11(7): 1243-1246.[19]Chen G, Qin SZ, Ma LT, et al. Zhonghua Qiguan Yizhi Zazhi. 2006;27(2):107-109.陈刚,秦尚振,马廉亭,等.成人骨髓间充质干细胞的培养及其向神经元样细胞诱导分化的实验[J].中华器官移植杂志,2006,27(2): 107-109.[20]Wang N, Yang SY, Zhang WZ, et al. Zhonghua Shenjing Waike Zazhi. 2005;21(1):55-58.王宁,杨树源,张文治,等.大鼠骨髓间充质干细胞体外诱导向神经元分化[J].中华神经外科杂志,2005,21(1):55-58.[21]Xiang J, Huang JF. Zhongguo Linchuang Jiepouxue Zazhi. 2012;30(5):587-589.向杰,黄继锋.人工神经导管基础与临床研究进展[J].中国临床解剖学杂志,2012,30(5):587-589.[22]Kehoe S, Zhang XF, Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury. 2012;43(5):553-572.[23]Huang JF, Zheng HY, Li SP, et al. Zhonghua Shouwaike Zazhi. 2009;25(3):154-157.黄继锋,征华勇,李世普,等.RGD多肽接枝聚复合导管桥接神经缺损的实验研究[J].中华手外科杂志,2009,25(3):154-157. [24]Dong HR, Xu YN, Huang JF. Zhongguo Linchuang Jiepouxue Zazhi. 2003;24(3):409-414.董红让,徐永年,黄继锋.聚乳酸/神经生长因子缓释导管修复周围神经缺损实验研究[J].中国临床解剖学杂志,2003,24(3): 409-414.[25]Tang F, Wang W. Zhonghua Sunshang yu Xiufu Zazhi: Dianzi Ban. 2009;4(1):97-101.汤锋,王伟.组织工程修复周围神经损伤的研究进展[J].中华损伤与修复杂志(电子版),2009,4(1):97-101.[26]Kokai LE, Lin YC, Oyster NM, et al. Diffusion of soluble factors through degradable polymer nerve guides: Controlling manufacturing parameters. Acta Biomater. 2009;5(7):2540- 2550.[27]Zhang WJ, Li BC, Wang JM, et al. Disan Junyi Daxue Xuebao. 2004;26(13):1165-1168.张文捷,李兵仓,王建民,等.组织工程人工神经诱导管的制备及其在体外与大鼠体内降解性能研究[J].第三军医大学学报,2004, 26(13):1165-1168.[28]de Ruiter GC, Malessy MJ, Yaszemski MJ, et al. Designing ideal conduits for peripheral nerve repair. Neurosurg Focus. 2009;26(2):E5. [29]Song M, Chen SZ, Han H, et al. Zhonghua Zhengxing Waike Zazhi. 2005;21(1):53-57.宋玫,陈绍宗,韩骅,等.微囊化转NGF基因3T3细胞修复大鼠周围神经损伤的实验研究[J].中华整形外科杂志,2005,21(1):53-57.[30]Wan ZT, Yin YX, Wang YH, et al. Wuhan Ligong Daxue Xuebao. 2010;32(12):19-21.万志涛,殷义霞,王永红,等.PNGF复合材料的神经生长因子缓释性能[J].武汉理工大学学报,2010,32(12):19-21. |